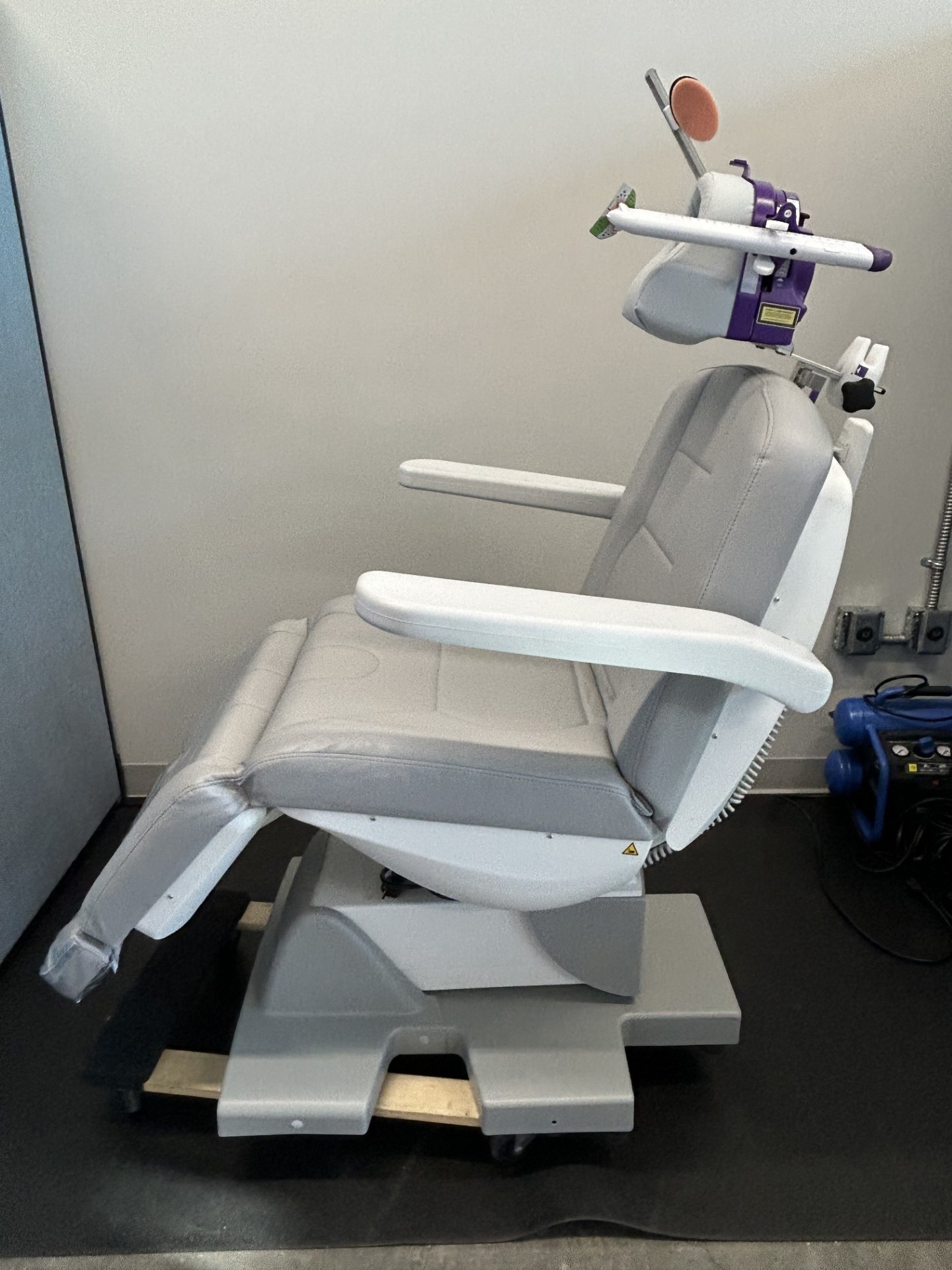
2022 Neuronetics NeuroStar

2022 Neuronetics NeuroStar
or
Call (612) 913-6373
Description
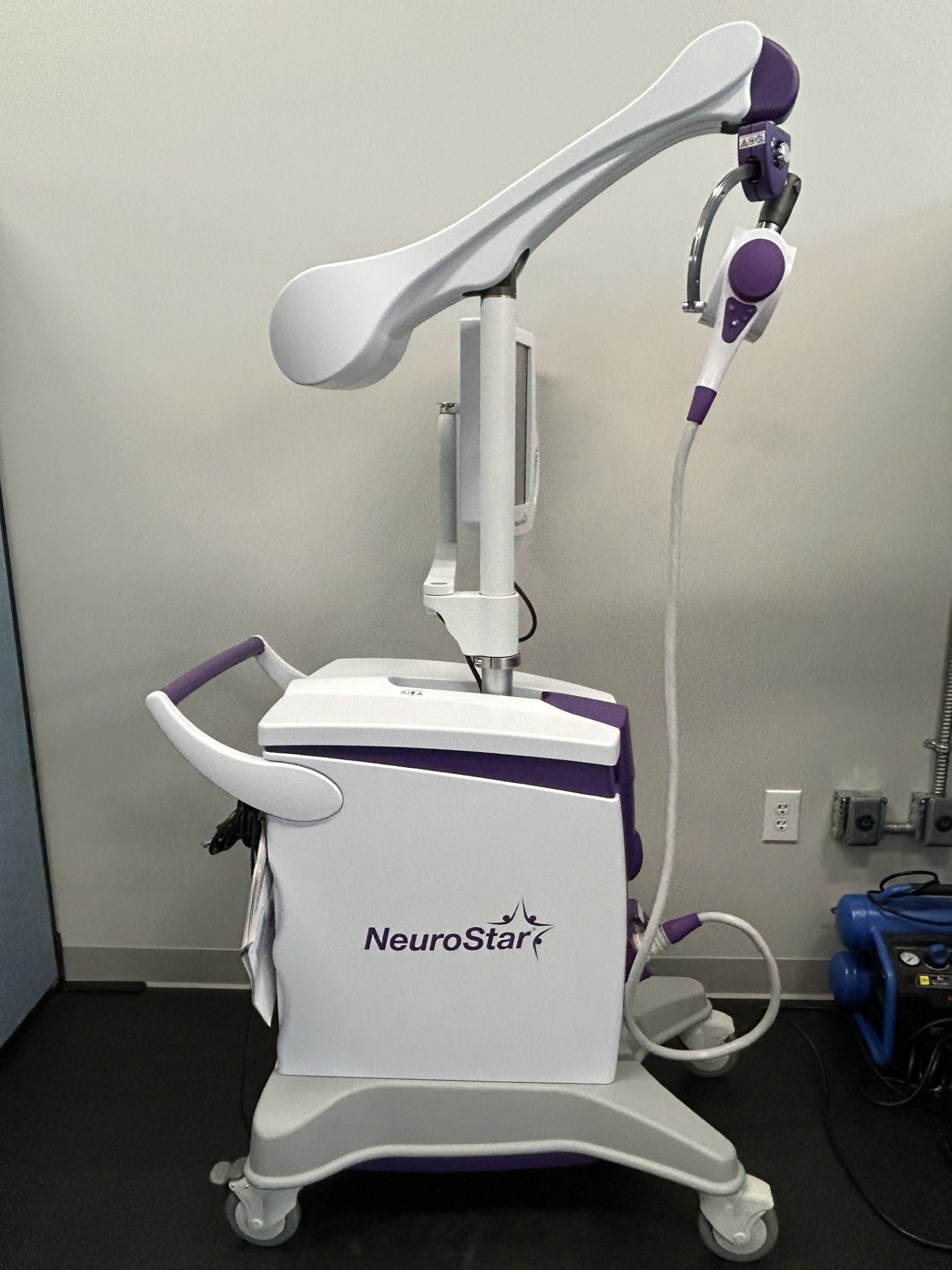
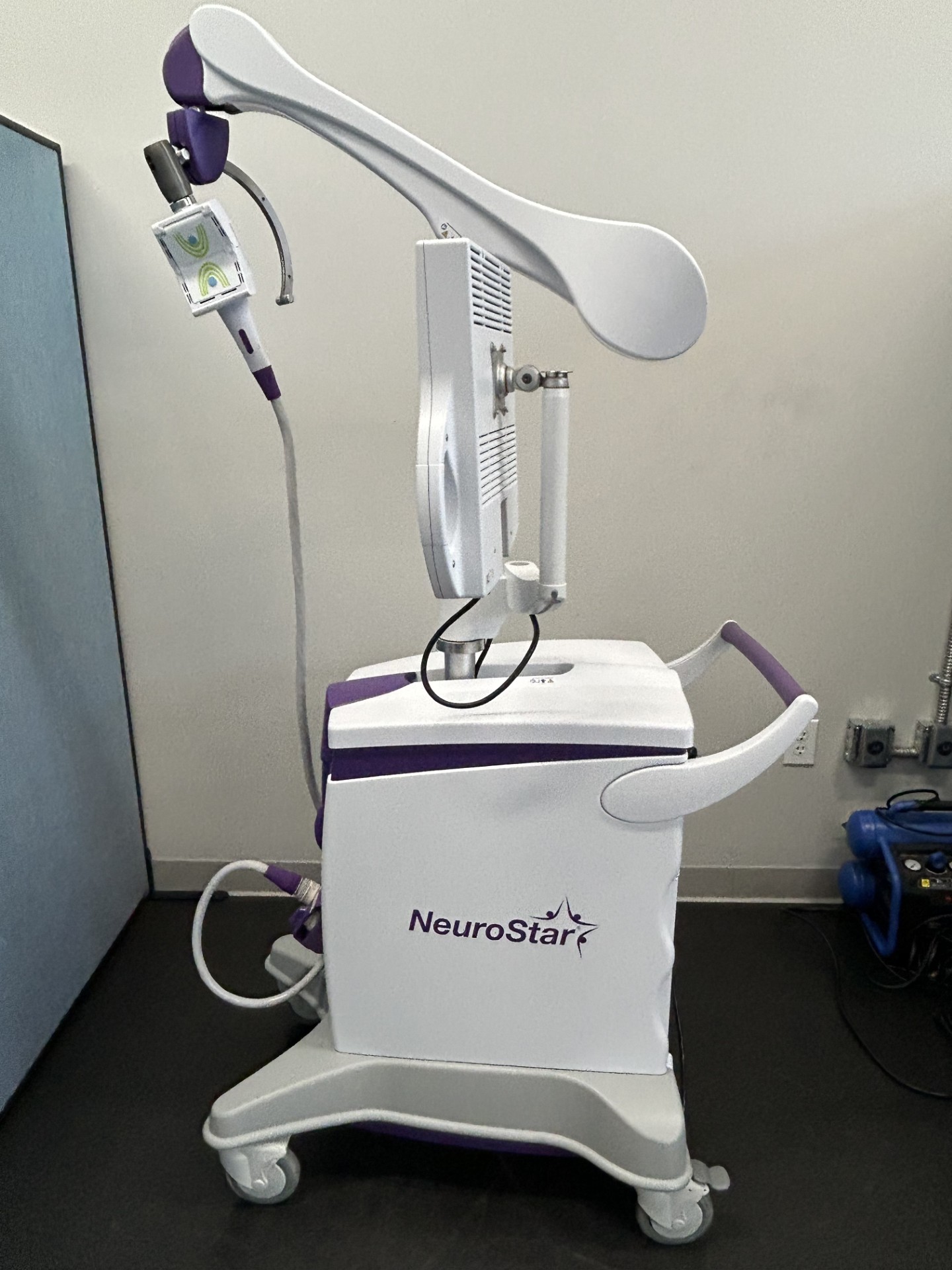
FDA-cleared, non-invasive, non-drug, in-office TMS (Transcranial Magnetic Stimulation) treatment that uses magnetic pulses to stimulate brain areas related to mood regulation. It is primarily used to treat adults with Major Depressive Disorder (MDD) who have not benefited from antidepressants, and was FDA-cleared in 2022 to treat anxious depression (MDD with anxious symptoms).
Key Uses and Indications for NeuroStar (as of 2022):
- Major Depressive Disorder (MDD): Treatment for adults who failed to achieve satisfactory improvement from prior antidepressant medication.
- Anxious Depression: Treatment to reduce anxiety symptoms in adults with MDD, cleared in 2022.
- Obsessive-Compulsive Disorder (OCD): Used as an adjunct treatment for OCD in adults.
- Adolescents: Indicated for MDD in patients aged 15-21.
Key Features and Effectiveness:
- Procedure: A non-invasive, outpatient, in-office procedure that typically takes 19-37 minutes per session.
- Effectiveness: In real-world studies, 83% of patients experienced a measurable reduction in depression severity, and 62% experienced full remission.
- Technology: Uses Contact Sensing technology to ensure precise, consistent dosage.
- Safety: Generally safe with minimal side effects, the most common being discomfort at the application site.
The 2022 TrakStar® system allows providers to manage treatment, including the updated protocols for anxiety, as part of the Neuronetics NeuroStar Advanced Therapy.
Specifications
| Manufacturer | Neuronetics |
| Model | Neuronetics NeuroStar |
| Year | 2022 |
| Condition | Used |
| Stock Number | SYS330 |